Generating strong immunity against infections and cancer
About 90% of humans have been exposed to the Epstein-Barr Virus (EBV) which contains a gene for the Latent Membrane Protein-1 (LMP1). Numerous studies have shown that LMP1 is a viral mimic of CD40, the receptor for CD40 ligand. Like the natural CD40 receptor, LMP1 can serve as the "ignition switch" for cellular and humoral immunity. Unlike the natural CD40 receptor, LMP1 starts up immunity without needing CD40 ligand because it is constitutively active.
Receptome's scientists have found that the DNA or RNA for LMP1 can be inserted into a wide variety of vaccine platforms as a "portable genetic adjuvant." LMP1 and its patented modifications are especially strong for activating dendritic cells (DCs) to generate CD8+ "killer" T cells against infectious agents and cancer cells. Because vigorous CD8+ T cell responses are especially challenging to generate using existing vaccine technologies, LMP1 can be used to fortify vaccines that would otherwise be too weak to be useful. Simply inserting LMP1 into these failed vaccines empowers them to become more effective, thereby salvaging their potential for protecting against HIV, influenza, and other viruses. In addition, LMP1 strongly activates dendritic cells to induce immunity against cancer cells.
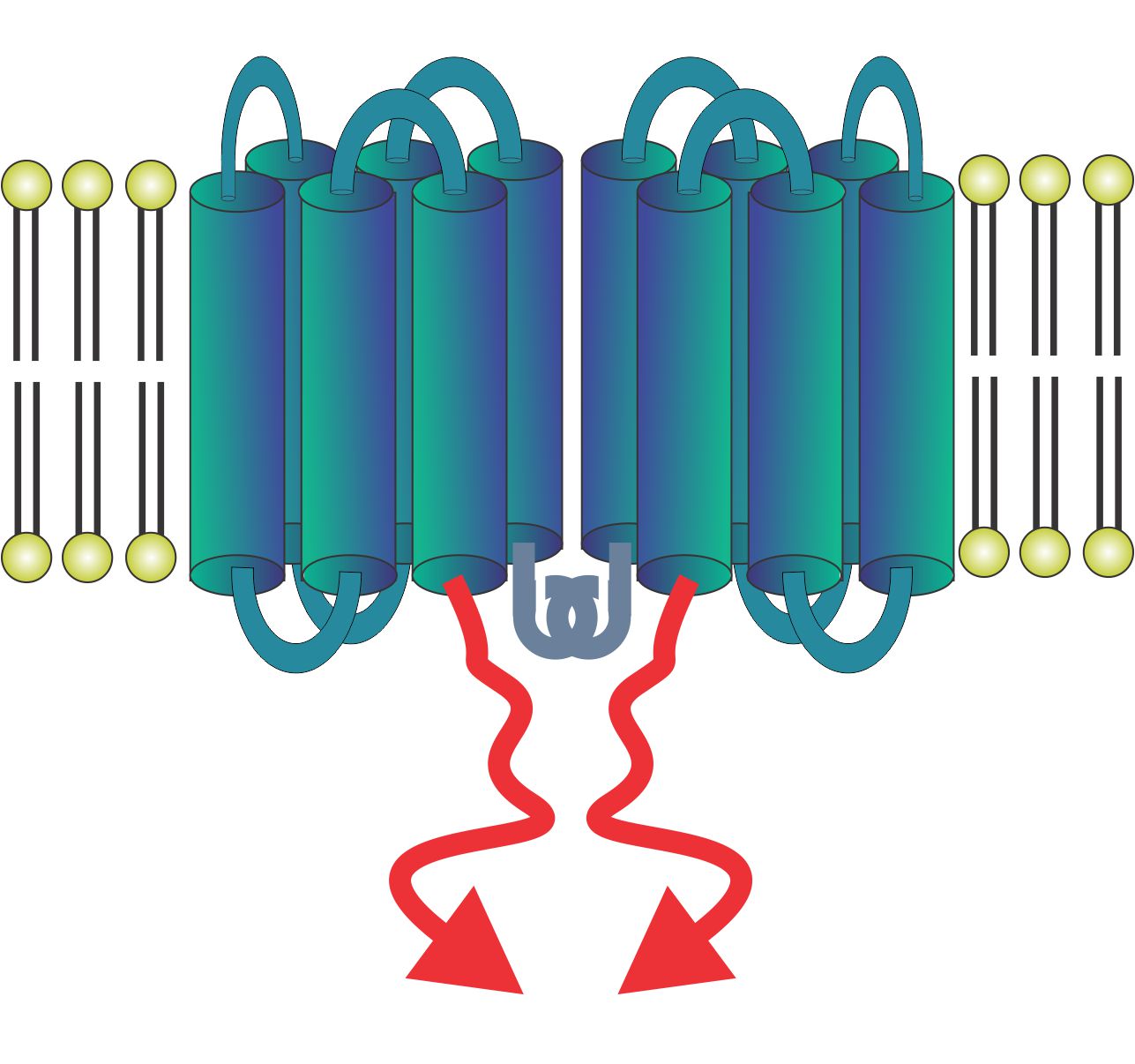
In addition, the N-terminal domain of LMP1 consists of 6 transmembrane domains and leads to aggregation of C-terminally located amino acids. This aggregation can be used to make constitutively activated forms of a number of important receptors. For example, the LMP-induced aggregation of IPS-1 leads to activation of the same pathway as STING. LMP-IPS-1 has significant potential as a vaccine adjuvant and cancer immunotherapy agent.