Technology
For many years, the late membrane protein-1 (LMP1) of Epstein-Barr Virus (EBV) was considered off-limits for biotechnology because it was assumed to be an oncogene responsible for EBV-associated cancers. More recently, LMP1 was shown to be insufficient to cause cancer (Hojer et al., Cancer Res. 74:4318-4328, 2014). On the contrary, LMP1 activates antigen-presenting cells to generate extraordinarily strong CD8+ T cell responses against other EBV proteins that control this virus for life (Zhang et al., Cell 148:739-751, 2012). It does so by serving as a viral mimic of CD40, a key activating receptor on B cells and dendritic cells (Graham et al., Immunol. Rev. 237:226-248, 2010). Based on these findings, the Kornbluth lab recognized that LMP1 could be used a powerful immune stimulant.
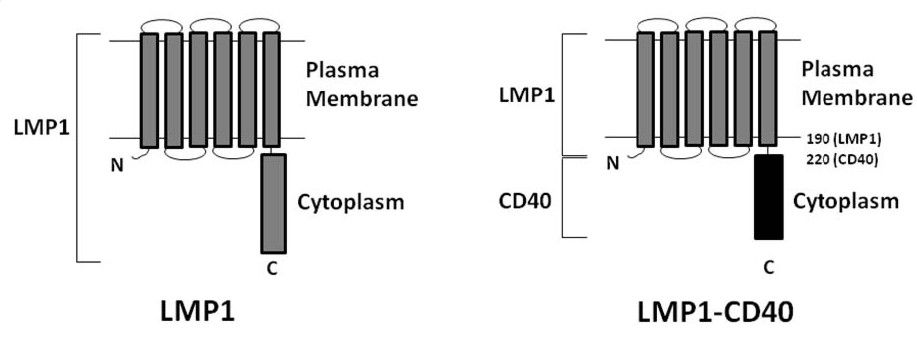
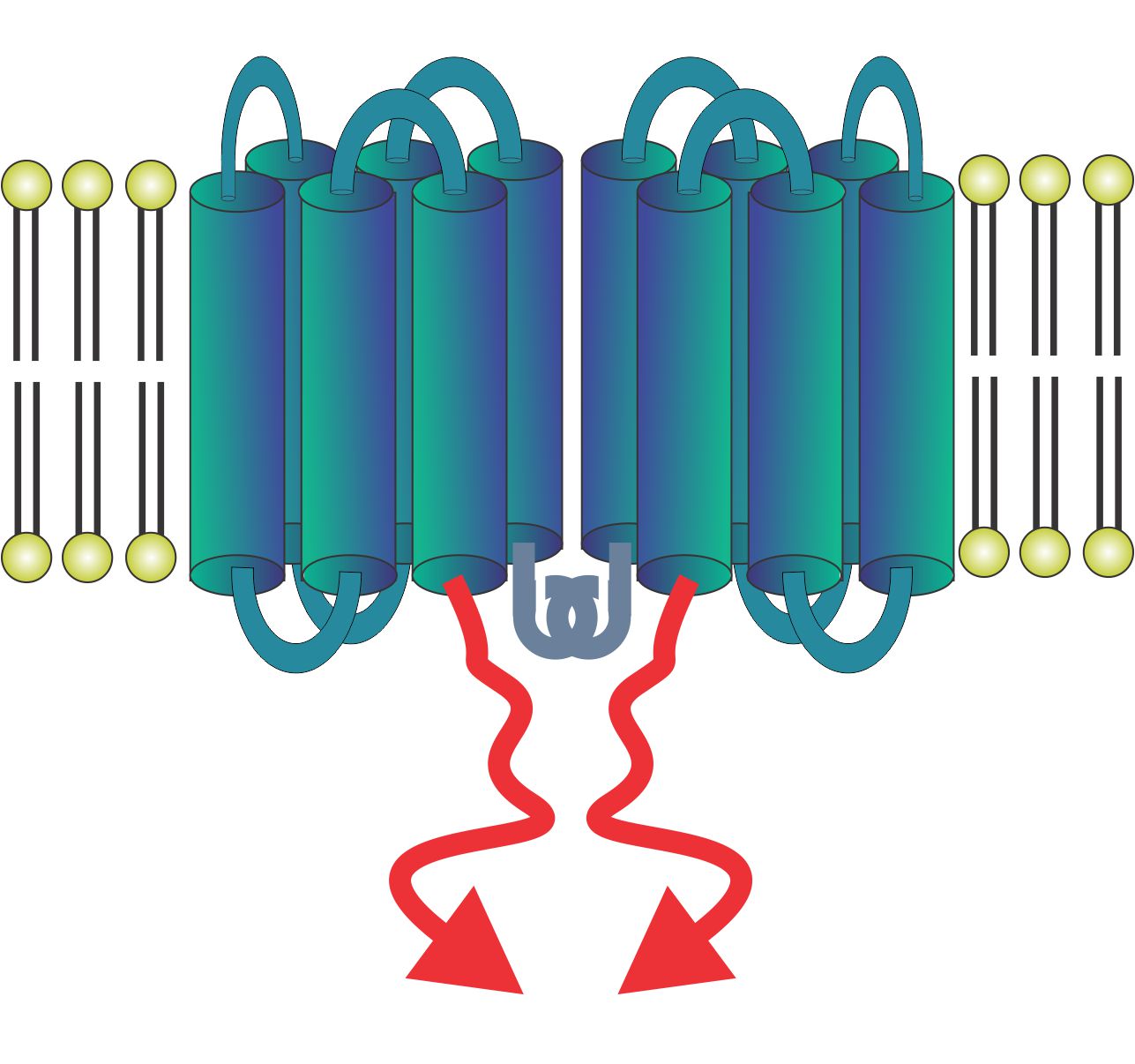
Structurally, LMP1 has six transmembrane domains that localize it to cell membranes. At the very N-terminus is a short sequence that brings many LMP1 molecules together into a clustered formation that promotes cell activation through the formation of supramolecular signaling complexes. The critical LMP1 intracellular domain binds to TRAFs, which are adaptor molecules that trigger downstream signaling events. Importantly, the LMP1 intracellular domain can be replaced by homologous intracellular domains from other receptors, such as CD40 itself to make LMP1-CD40.